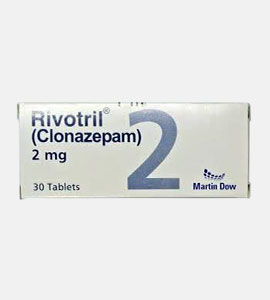
Klonopin (Clonazepam)
* Alleen ter illustratie
Klonopin (Clonazepam)
Korte omschrijving
Klonopin (Clonazepam) 2mg (UK) 30pillen 30 pillen
2,820.01 NOK
Klonopin (Clonazepam) 2mg (UK) 60pillen 60 pillen
4,121.55 NOK
Klonopin (Clonazepam) 2mg (UK) 90pillen 90 pillen
5,411.67 NOK
Klonopin (Clonazepam) 2mg (UK) 120pillen 120 pillen
6,713.22 NOK
Klonopin (Clonazepam) 2mg (UK) 180pillen 180 pillen
9,316.30 NOK
Klonopin (Clonazepam) 2mg (UK) 240pillen 240 pillen
11,907.97 NOK
Klonopin (Clonazepam) 2mg (UK) 270pillen 270 pillen
13,209.51 NOK
Klonopin (Clonazepam) 2mg (UK) 360pillen 360 pillen
17,102.72 NOK
Klonopin (Clonazepam) 2mg (EU) 30pillen 30 pillen
2,112.15 NOK
Populair Klonopin (Clonazepam) 2mg (EU) 60pillen 60 pillen
2,877.09 NOK
Klonopin (Clonazepam) 2mg (EU) 90pillen 90 pillen
3,642.03 NOK
Klonopin (Clonazepam) 2mg (EU) 120pillen 120 pillen
4,406.97 NOK
Klonopin (Clonazepam) 2mg (EU) 180pillen 180 pillen
5,948.27 NOK
Klonopin (Clonazepam) 2mg (EU) 240pillen 240 pillen
7,478.16 NOK
Klonopin (Clonazepam) 2mg (EU) 270pillen 270 pillen
8,243.10 NOK
Klonopin (Clonazepam) 2mg (EU) 360pillen 360 pillen
10,537.92 NOK
toegevoegd aan winkelwagentje
-
Verzending5-7 dagenGegarandeerde levertijd 16 dagenGegarandeerde levertijd 16 dagenGenoemde doseringen (EU), worden binnen Europa verzonden en garanderen een snellere levering.
-
Betaling






-
Upgrade en bespaar. Voeg items aan je winkelwagentje toe en krijg gratis verzending
2283.41 NOK




Klonopin (Clonazepam)
Regarding Shipping: Products with dosages mentioned (EU), are shipped inside Europe and garantuee faster time in delivery.